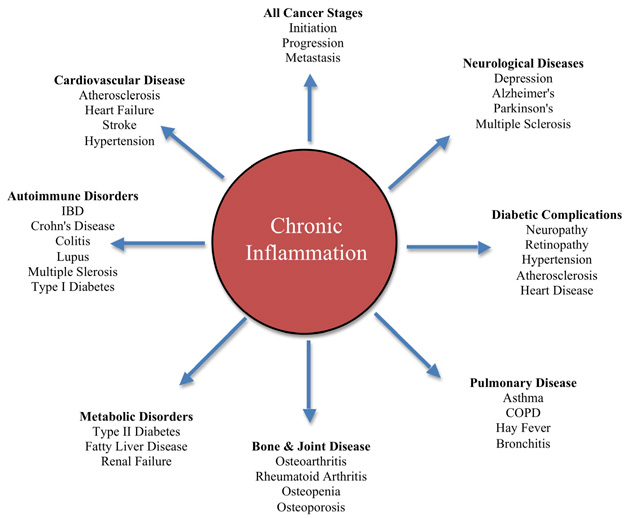
Inflammation is the underlying cause to the aging process. As we age, our immune system produces what is known as “low-grade” inflammation.
For many, inflammation is simply understood as a trajectory of biomarkers, for example the appearance of IL-6 or C-reactive protein (CRP), associated with a disease. However, inflammation is a very complex response to an injury, infection, or other stimulus, in which many different cells types and secreted factors orchestrate protective immunity, tissue repair, and resolution of tissue damage. Whereas acute inflammation limits tissue damage and resolves, chronic prolongation of the inflammatory state leads to progressive tissue damage. A central question, then, is how do we describe and begin to understand the mild pro-inflammatory state of aging. Luigi Ferrucci (NIA, NIH)
Inflammation is correlated with Diabetes II, obesity and cardiovascular disease, several cancers, frail bones, etc. So, what is the connection to telomeres?
There are several considerations in relating telomere biology to aging. First, physiologically there is overlap between the shortest telomere length of young children and the longest telomeres of the elderly. Most telomere shortening occurs early in life, in association with growth, and when the rate of disease in general is low. The paradigmatic telomere syndrome of dyskeratosis congenita is not at all typical of progerias, inherited syndromes in which patients appear old and suffer diseases of aging such as premature atherosclerosis or dementia. Furthermore, the organ damage of dyskeratosis congenita is not very similar to normal aging of marrow, lungs, and liver. The marrow becomes mildly hypocellular in older individuals, but stem cell numbers may actually increase, and blood counts remain stable; and neither the liver nor lungs normally become fibrotic with advanced age, as they often do in dyskeratosis congenita patients. Although in adults, relatively short leukocyte telomeres have been associated with cardiovascular events—a common morbidity of the aging population—the clinical correlations have not been consistent, and may be related to overall reactive oxygen species exposure. Rodrigo Calado and Neal Young – The Scientist, May, 2012
Epigenetics and Inflammation
Epigenetic or phenotype modifications are done through gene expression. The most well known of these is DNA methylation. The methylation pathway is the super highway for inflammation.
Methylation is the process of adding a carbon and three hydrogen atoms to a molecule. Neurotransmitters and proteins get methylated. The body uses methylation to create T-cells to kill viruses, bacteria, and cancers. It is a series of over 100 reactions in the body that are responsible for production of dopamine, growth factors, and glutathione. Glutathione helps with removal of heavy metals. When methylation is impaired, B-cells will be high and you will have ongoing inflammation. Viruses create cytokine storms which also propogate inflammation. You simply cannot heal while you are inflamed. Once methylation is corrected, inflammation generally reduces.
Here is a graphic of how cigarette smoke impairs methylation:
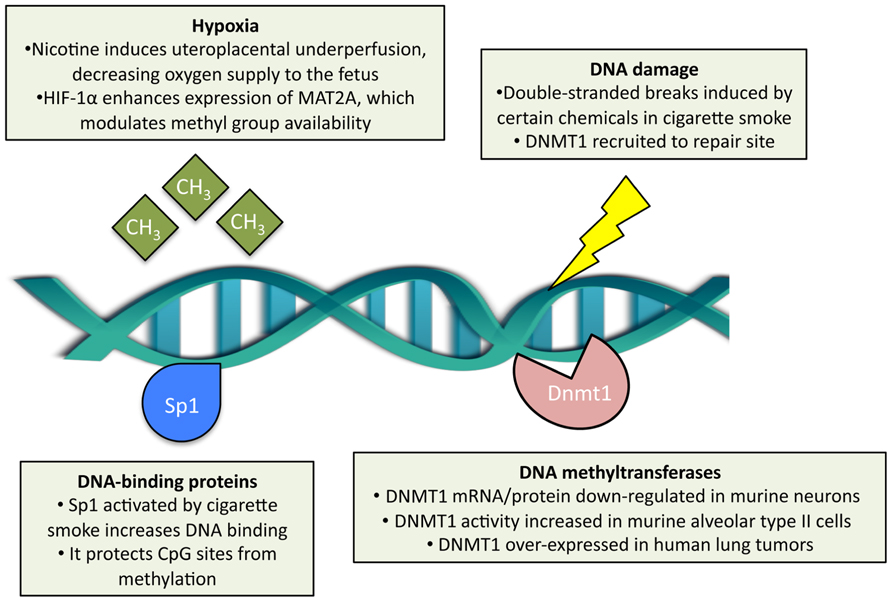
DNA methylation is the most studied epigenetic modification, capable of controlling gene expression in the contexts of normal traits or diseases.
Healthy function of telomeres requires adequate methylation. The important point to understand is that an adequate supply of methyl donors is needed for telomeres to work properly, just like your car needs gasoline.
In Part 3 we will examine epigenetic factors to help fix methylation pathways as a key component to the aging process and the various testing options for methylation and other related diagnostics.